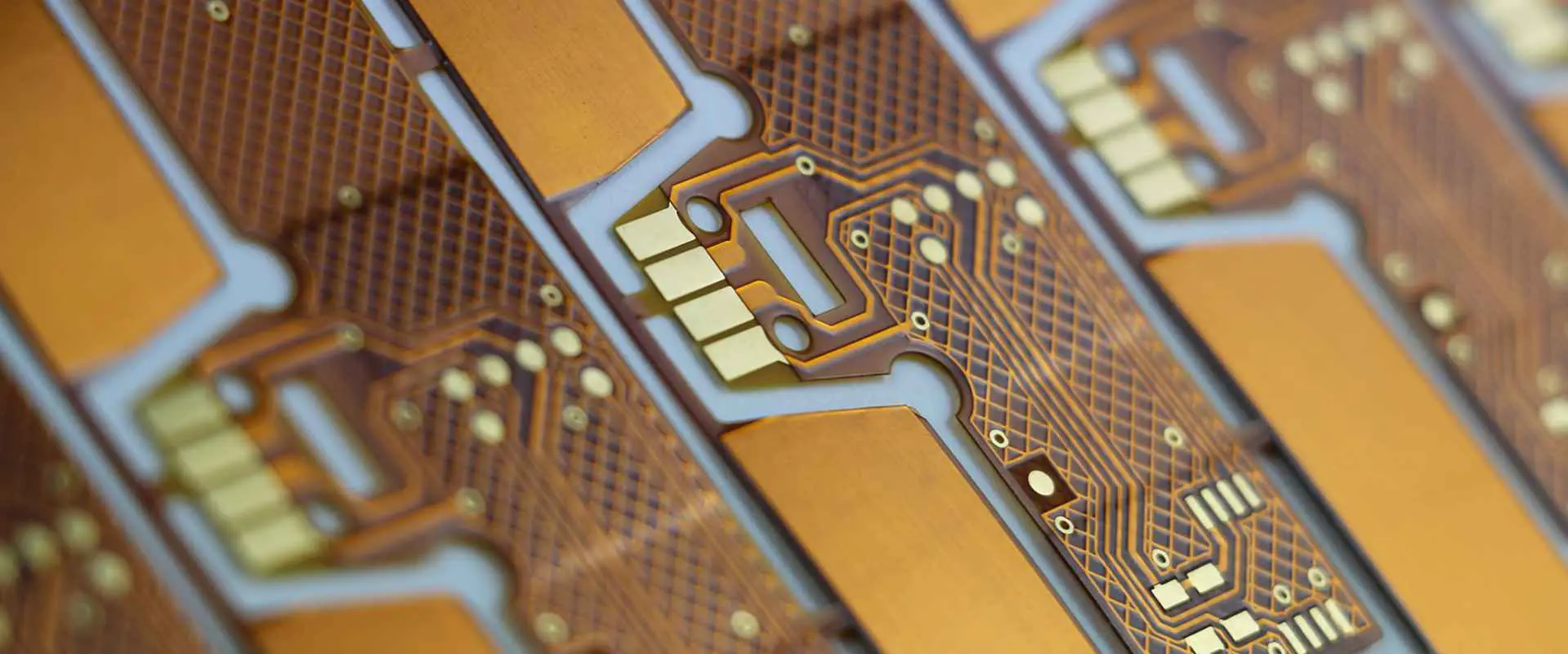
Our medical grade flexible printed circuit boards (Flex PCBs) are engineered specifically for demanding healthcare and biomedical applications where reliability, biocompatibility, and precision are paramount. These ultra-thin, bendable circuits enable innovative medical device designs while meeting the strictest regulatory standards for patient safety.
Key Features:
Biocompatible Materials – Manufactured using FDA-approved substrates and materials that meet ISO 10993 biocompatibility standards, ensuring safe direct or indirect patient contact without adverse biological reactions.
Superior Flexibility – Dynamic flex capabilities withstand millions of bend cycles, making them ideal for wearable medical devices, implantable electronics, and equipment requiring constant movement or positioning adjustments.
Miniaturization Excellence – Ultra-compact designs enable smaller, lighter medical devices while maintaining high circuit density and performance. Thickness options from 0.05mm to 0.5mm accommodate space-constrained applications.
Enhanced Reliability – Rigorous quality control processes and specialized manufacturing techniques ensure consistent performance in critical medical environments. Zero-defect tolerance for life-critical applications.
Chemical Resistance – Robust protection against sterilization processes including gamma radiation, ethylene oxide, and autoclave sterilization methods commonly used in medical facilities.
High-Frequency Performance – Optimized for medical imaging, monitoring, and diagnostic equipment requiring precise signal integrity and minimal electromagnetic interference.
Applications:
- Implantable medical devices (pacemakers, defibrillators, neural stimulators)
- Wearable health monitors and fitness trackers
- Surgical instruments and robotic systems
- Medical imaging equipment (MRI, ultrasound, X-ray)
- Patient monitoring systems
- Hearing aids and cochlear implants
- Catheter and endoscope electronics
Compliance & Certifications:
- ISO 13485 medical device quality management
- FDA CFR 21 Part 820 compliance
- IPC-6013 flexible printed circuit standards
- RoHS and REACH compliant materials
Custom Engineering Services: Our medical device engineering team provides comprehensive design support, prototype development, and regulatory guidance to accelerate your product development timeline while ensuring full compliance with medical device regulations.
Transform your medical device concepts into reality with flexible circuits designed for the unique challenges of healthcare technology.