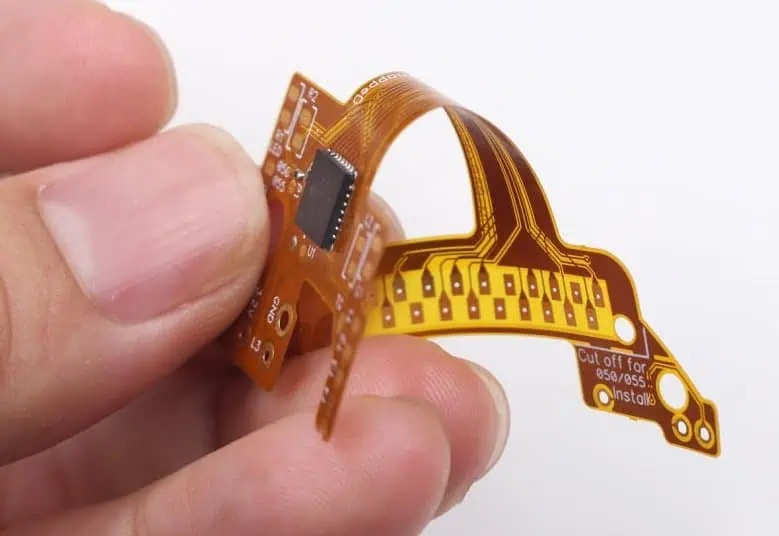
Our Medical Grade Flex PCBs deliver the precise performance and uncompromising safety required for today’s most demanding medical devices. Engineered with biocompatible materials and manufactured under strict quality controls, these flexible circuits enable innovative medical technology while meeting the highest regulatory standards.
Key Features:
Biocompatible Construction – Manufactured using USP Class VI certified materials and biocompatible adhesives that meet ISO 10993 standards for biological evaluation of medical devices. Safe for direct and indirect patient contact applications.
Medical-Grade Materials – Premium polyimide substrates with specialized copper traces designed for long-term reliability in medical environments. Resistant to sterilization processes including ETO, gamma radiation, and autoclave cycles.
Ultra-Thin Profile – Flexible circuits as thin as 0.05mm enable integration into space-constrained medical devices while maintaining excellent electrical performance and mechanical durability.
High Reliability – Rigorous testing protocols ensure consistent performance in critical applications. Features excellent fatigue resistance for devices requiring repeated flexing during operation.
Regulatory Compliance – Manufactured in ISO 13485 certified facilities with full traceability and documentation to support FDA submissions and CE marking requirements.
Applications:
- Implantable medical devices and pacemakers
- Surgical instruments and endoscopes
- Patient monitoring systems
- Hearing aids and cochlear implants
- Diagnostic equipment and imaging systems
- Wearable health monitoring devices
Specifications:
- Layer count: 1-8 layers
- Minimum trace width: 0.05mm (2 mil)
- Operating temperature: -40°C to +125°C
- Dielectric strength: >3000V
- Flex life: 100,000+ cycles
Trust our Medical Grade Flex PCBs to deliver the performance, reliability, and safety your medical devices demand. Contact our engineering team for custom design consultation and rapid prototyping services.